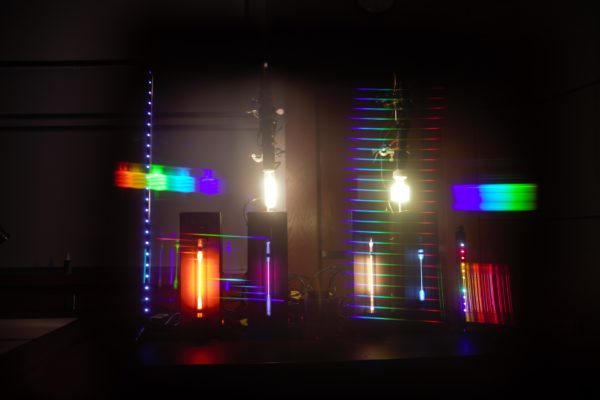
(7B10.10) Emission Spectra
$0.00
Certain gasses absorb and emit different wavelengths of light that are characteristic to only that element. By applying a voltage through a tube with a gas, such as hydrogen or neon, you can see their individual emission spectrums and the colors they do and don’t emit. You can compare that to the continuous spectrum seen from a lightbulb.








Reviews
There are no reviews yet.